The general rules for writing Lewis structures include the following. Lewis structures for most molecules can be drawn by following a simple strategy.
In short these are the steps you need to follow for drawing a Lewis structure.
. 1Using your rules for drawing Lewis Dot Structures draw the following molecules. Add up the valence electrons of the atoms. Group number for each element of valence electrons.
Molecule Dot Structure VSEPR shape CF4 Cl20 C2H4 HCN HF PF. Subtractelectrons for positively charged ions. H is NEVER a center atom.
In writing Lewis structures for covalent compounds you must remember the following. Dont be concerned with which atom gave what. Determine the total number of valence electrons.
WRITING LEWIS DOT STRUCTURES Lewis structure or formula shows electrondot symbols for the atoms the bonding pairs as lines and the lone pairs that fill each atoms outer level valence shell as pairs of dots. Instructions for Drawing Lewis Structures. Draw Lewis structures for atoms ions and simple molecules.
Determine the total number of valence electrons in the molecule. Draw a lewis structure for ketene c2h2o which has a carboncarbon double bond. Determine the total number of valence electrons in the molecule or polyatomic ionSimply.
The sum of the number of valence electrons is equal to N. Addelectrons for negatively charged ions. The general rules for drawing Lewis structures are given below.
All atoms have their correct charge. All valence electrons of the atoms in Lewis structures must be shown. For cations subtract one electron for each unit of positive charge.
It should be the most symmetric structure you can draw. Make sure that H has only 1 bond and that the other atoms do not exceed their normal number 2 bonds to O 3 bonds to N 4 bonds to C and that no atom in the molecule has more than 4 bonds. LEWIS STRUCTURES AND MOLECULAR SHAPE D.
The general rules for drawing Lewis structures are given below. Write the correct skeletal structure for the molecule. Drawing Lewis Structures A step-by-step Guide by C.
When electrons are shared the bond is called a covalent bond. Draw the initial skeletal structure with atoms connected by single bonds. Determine the total number of valence electrons.
Generally electrons are paired. A polyatomic anion or cation add. The least electronegative element goes in the center.
And recall that for main group elements the valence electrons can be determined from the group number. The formula for water is H 2 O. Determine the electron and molecular geometry of the produced molecules.
The number of electrons shared in the molecule equals S. ISOMERS AND RESONANCE STRUCTURES Complete the Table below providing the suggested. For ions the charge must be taken into account.
Rules for drawing Lewis dot structures Count the number of valence e- each atom brings into the molecule. For molecules and polyatomic ions composed of nonmetals. Make use of the N A S equation to get to know the number of bonds.
Determine the total number of valence electrons in the molecule or polyatomic ionSimply. Writing Lewis Dot Structures. The number of valence electrons in electron pot is A.
Count the total number of valence electrons in the structure Remember Group of Valence electrons 2. Draw the lewis dot structure. Determine the electron and molecular geometry of the produced molecules.
Molecule Dot Structure VSEPR shape CF4 Cl20 C2H4 HCN HF PF. Ill cover how to properly draw lewis structures of regular. Draw an acceptable lewis dot structure for each of the following.
Hydrogen atoms are always terminal only one bond Put more electronegative elements in terminal positions. When electrons are transferred the bond is called an ionic bond. Unpaired electrons are observed in odd electron molecules such as NO and NO2.
LEWIS STRUCTURES General Rules for Drawing Lewis Structures 1. Put electron pairs about each atom such that there are 8 electrons around each atom. Scientists often create models to represent either a physical or abstract system or structure that.
The SUM TOTAL is what is important. Decide on a skeletal structure What is bonded to what The central atom is generally written first in the formula Hydrogen is never the central atom even if it is. Use Lewis structures as a guide to construct three-dimensional models of small molecules.
Count the total number of valence electrons in the molecule or polyatomic ion. MUST FOLLOW IN ORDER. Exceptions to the Octet Rule Non-bonding Bonding e clouds e clouds on Molecule Lewis Structure on central atom central atom BF 3 e pairs ShapeGeometry PCP 56 pairs SF 6 epairs IF 5 pairs XeF6 6 pairs EXPERIMENT 6.
Rules for Writing Lewis Structures. If the entire molecule is charged ie. Counting valence electrons yields eight total six from oxygen one each from the two hydrogens.
Count the total number of valenceelectrons. Rules for Drawing Lewis Structure. Distribute the electrons from electron pot to account for the bonds.
For example H 2 O has 2x1 6 8 valence electrons CCl 4 has 4 4x7 32 valence electrons For anions add one valence electron for each unit of negative charge. In chemical bonds atoms can either transfer or share their valence electrons. Drawing Lewis Dot Structures for Molecules Background Information.
Lewis Dot Structure Worksheets - Thekidsworksheet Lewis structures practice worksheet draw the lewis structures for each of the following molecules. To do this you will need the periodic table. General rules for writing LEWIS structures for molecules.
Sum the valence electrons from all the atoms. 1Using your rules for drawing Lewis Dot Structures draw the following molecules. Molecule Dot Structure VSEPR shape CF4 Cl20 C2H4 HCN HF PF.
Hydrogen is special since it can only accommodate a duet and therefore can form at most one bond. Identify the number of valence electrons and draw the lewis dot structure notes. Hoeger 1 Determine the total number of valence electrons for ALL atoms.
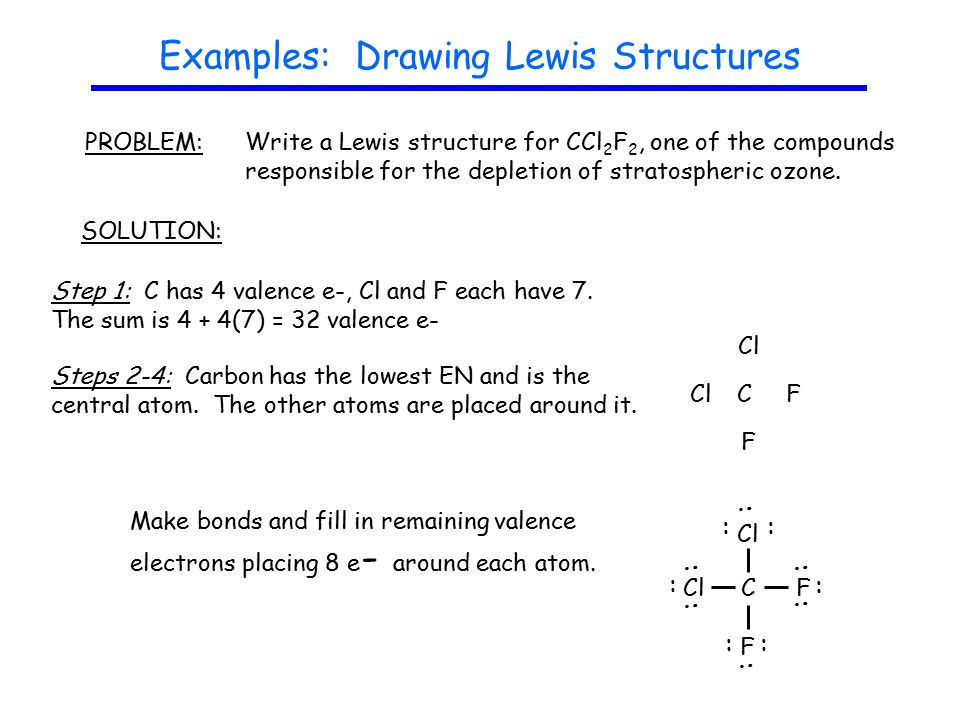
Guidelines Drawing Lewis Structures Ppt Video Online Download

Objectives I Can Draw Simple Lewis Structures Of Molecules Ppt Download
Lewis Electron Dot Structures Help
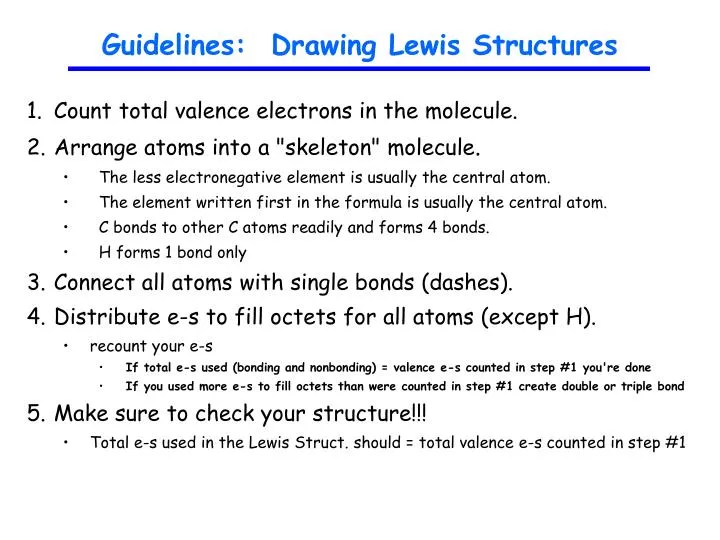
Ppt Guidelines Drawing Lewis Structures Powerpoint Presentation Free Download Id 1812949

Electron Dot Model Of Bonding Lewis Structures

Drawing Lewis Structures A Tutorial On Writing Lewis Dot Structure Ppt Video Online Download


0 comments
Post a Comment